HiPerFlon PTFE / PFA Lined Piping Systems
Corrosion Resistant Lined Piping in ASME and DIN piping classes.
Read more
If you can't find what you're looking for here, don't hesitate to contact us.
+44 (0) 1706 756 400 enquiry@crp.co.ukCorrosion Resistant Products Ltd, Todmorden Road, Littleborough, UK,
OL15 9EG

If you can't find what you're looking for here, don't hesitate to contact us.
+44 (0) 1706 756 400 enquiry@crp.co.ukCorrosion Resistant Products Ltd, Todmorden Road, Littleborough, UK,
OL15 9EG
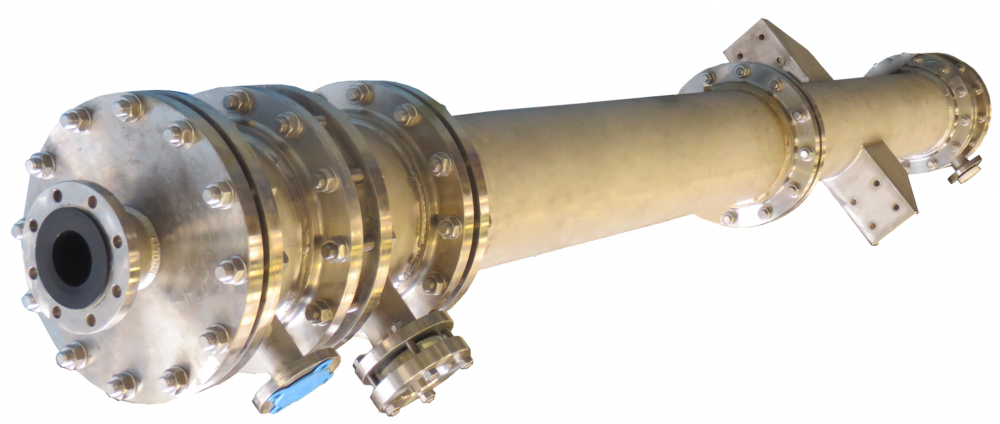
If you can't find what you're looking for here, don't hesitate to contact us.
+44 (0) 1706 756 400 enquiry@crp.co.ukCorrosion Resistant Products Ltd, Todmorden Road, Littleborough, UK,
OL15 9EG

If you can't find what you're looking for here, don't hesitate to contact us.
+44 (0) 1706 756 400 enquiry@crp.co.ukCorrosion Resistant Products Ltd, Todmorden Road, Littleborough, UK,
OL15 9EG
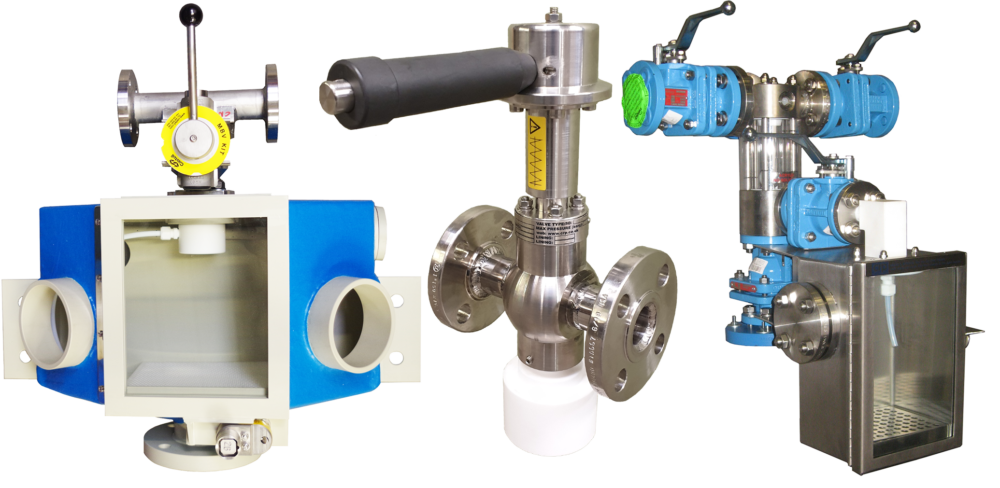
If you can't find what you're looking for here, don't hesitate to contact us.
+44 (0) 1706 756 400 enquiry@crp.co.ukCorrosion Resistant Products Ltd, Todmorden Road, Littleborough, UK,
OL15 9EG
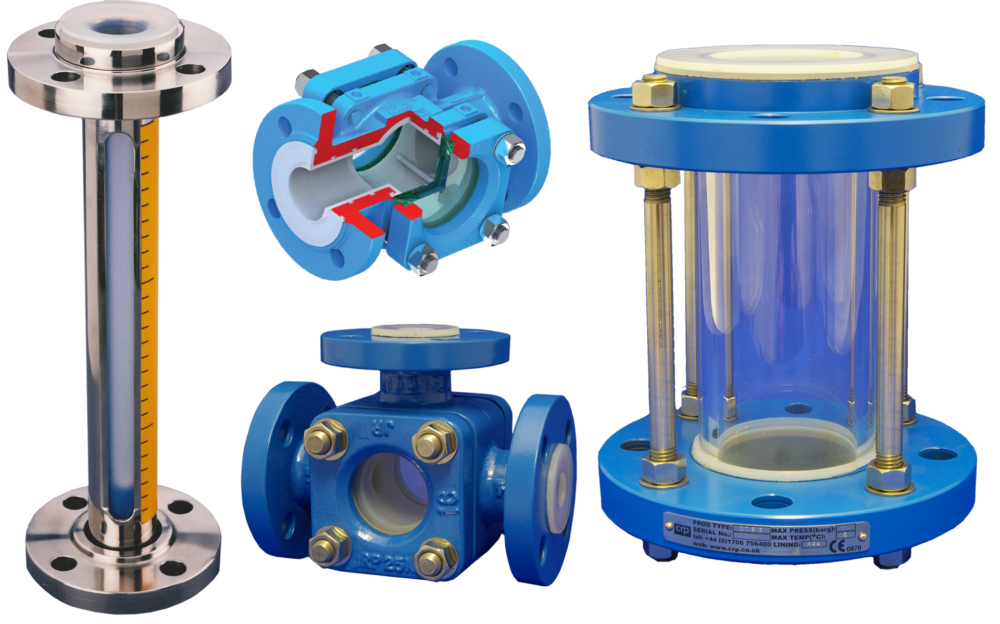
If you can't find what you're looking for here, don't hesitate to contact us.
+44 (0) 1706 756 400 enquiry@crp.co.ukCorrosion Resistant Products Ltd, Todmorden Road, Littleborough, UK,
OL15 9EG

If you can't find what you're looking for here, don't hesitate to contact us.
+44 (0) 1706 756 400 enquiry@crp.co.ukCorrosion Resistant Products Ltd, Todmorden Road, Littleborough, UK,
OL15 9EG

If you can't find what you're looking for here, don't hesitate to contact us.
+44 (0) 1706 756 400 enquiry@crp.co.ukCorrosion Resistant Products Ltd, Todmorden Road, Littleborough, UK,
OL15 9EG
CRP can supply our products around the world, we also have an established network of distributors and agents who are able to provide you with local service and support, please contact your local representative for information and service. Many of our distributors carry stock of our products ready to assist you.